· cybersecurity · 7 min read
The Role of IV&V (Independent Verification and Validation) in Modern Compliance: Why It Matters for Your Organization
Independent Verification and Validation (IV&V) is essential in corporate governance and regulatory compliance, ensuring organizational processes and systems exceed industry standards. IV&V, by its design, serves as a critical audit function, providing a third-party perspective that is important in identifying discrepancies that internal reviews might overlook.
Independent Verification and Validation (IV&V) is essential in corporate governance and regulatory compliance, ensuring organizational processes and systems exceed industry standards. IV&V, by its design, serves as a critical audit function, providing a third-party perspective that is important in identifying discrepancies that internal reviews might overlook.
In today’s complex regulatory environment, merely adhering to compliance standards is no longer sufficient. As businesses incorporate increasingly sophisticated technologies, the complexity of systems escalates, heightening the risk of compliance failures. IV&V of critical software[1] acts as a safeguard, a methodological approach that independently assesses whether systems are functioning as intended and in compliance with all regulatory requirements. This is particularly vital in industries such as healthcare where the cost of non-compliance can result in severe financial penalties and reputational damage.
What is Independent Verification and Validation of Software?
In modern software verification and validation (V&V), the primary goals are to identify and resolve issues related to software, hardware, and system components early in the development lifecycle. Verification involves activities that ensure the software being developed matches the specified requirements. Validation, on the other hand, focuses on confirming that the system meets its intended operational objectives. The distinction between verification and validation is often blurred, and software practitioners tend to approach them as a unified process aimed at ensuring the software performs as expected. In general, Independent Verification and Validation (IV&V) has three main objectives:
- identifying issues early,
- ensuring requirement adherence, and
- confirming operational readiness

Strategic Advantages of IV&V
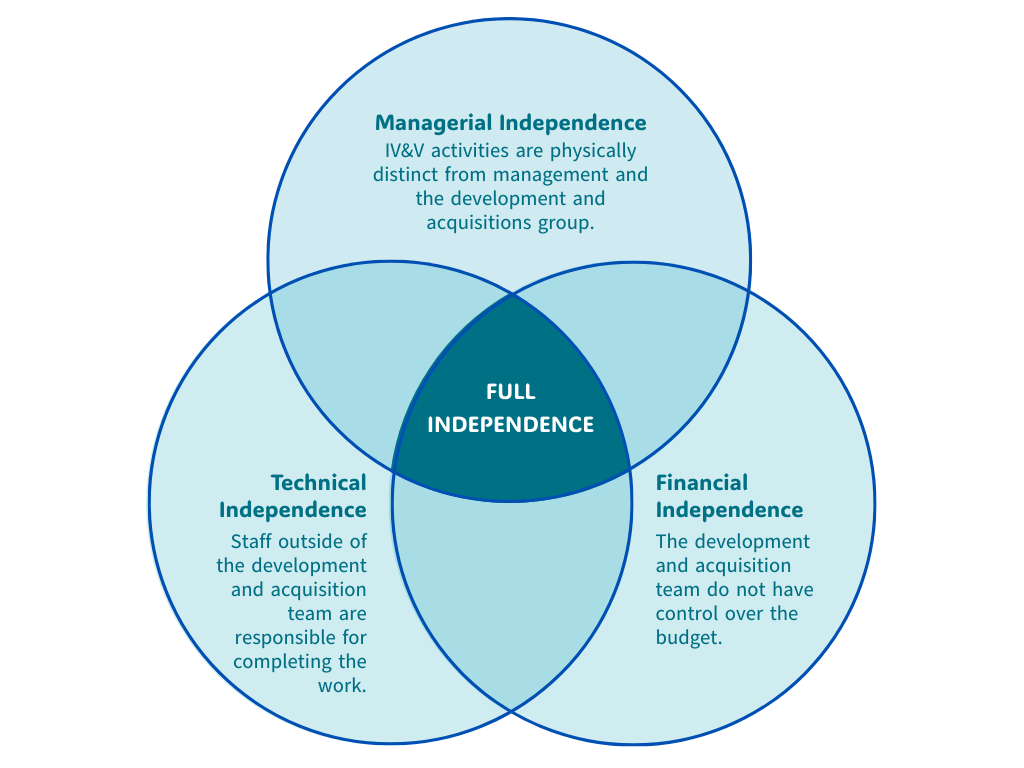
IV&V providers operate independently from the development and operational teams. This separation is necessary as it eliminates potential biases and ensures an objective assessment, leading to more accurate validation of compliance with standards like ISO[2], GDPR[3], or HIPAA[4].
By identifying non-conformities and potential failures before they become systemic, IV&V helps organizations pre-emptively address issues that could lead to non-compliance. This proactive approach not only saves costs associated with regulatory fines but also avoids the downtime required to rectify compliance breaches.
For stakeholders, the assurance that an independent body[5] has verified and validated the compliance of products or systems builds trust[6]. This is especially significant for public companies, healthcare providers, and entities in the financial sector where maintaining stakeholder trust is paramount.
The Critical Role of IV&V in Compliance
Regulatory bodies are increasingly recognizing the importance of IV&V in compliance frameworks. For example, in the healthcare sector[7], where patient data security is critical, IV&V can ensure that data handling systems comply with privacy laws and regulations, thus safeguarding sensitive information against breaches and unauthorized access.
Moreover, in the aerospace[8] and defense industries[9], where safety and functionality are critical, IV&V provides an assurance[10] that the systems will perform reliably under extreme conditions. This not only complies with international safety standards but also ensures the operational readiness and reliability of critical defense infrastructure.
In software development, Independent Verification and Validation (IV&V) has proven effective in identifying errors and deficiencies that might otherwise be missed. The presence of a capable IV&V team incentivizes developers to maintain high standards in both development and maintenance processes. However, the effectiveness of IV&V findings is closely linked to how integrated the IV&V team is with the development team—the more detached the IV&V organization is, the slower the feedback loop to the developers. Typically, everyone involved in writing requirements, coding, or testing is engaged in verification and validation (V&V), including software developers.
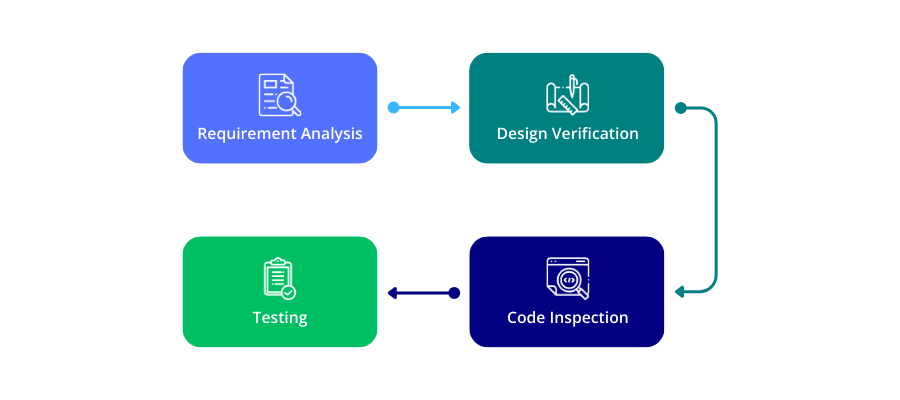
Independent Verification and Validation (IV&V) activities generally target either the software development process or the products resulting from that process. Process-oriented IV&V includes participating in systems and software requirements reviews, conducting design and code inspections, and monitoring and auditing tests. This technical review ensures that standards and procedures are adhered to within the development environment. Product-oriented IV&V involves an independent analysis of the developer’s products — such as system and software requirements, design, code, and test plans — and includes independent testing and test planning for both the software as a standalone item and as part of the entire system. This can occur during development and after delivery.
Typically, IV&V implementations combine both process-oriented and product-oriented assurance. Exclusively focusing on the development process without a detailed technical review of the product does not ensure quality. For large, complex systems involving multiple development organizations and numerous software and system interfaces, it’s impossible to foresee all issues that a process review must address. This could lead to overlooked issues unless the software products are continually reviewed and integrated. Conversely, maintaining high-quality products is challenging without a high-quality process. Moreover, an exhaustive product review is often too costly, making process reviews essential for added confidence in product quality.
Why IV&V Should Be Part of Your Compliance Strategy
Organizations that implement IV&V demonstrate a commitment to quality and continuous improvement, setting them apart in competitive industries. Moreover, the independent nature of IV&V fosters a transparency that can significantly improve your brand’s credibility and trustworthiness.
As the regulatory landscape continues to expand and change, the importance of IV&V in ensuring compliance cannot be overstated. It provides a clear, objective, and thorough evaluation that is crucial for any organization aiming to not only meet but exceed today’s stringent compliance requirements. By embedding IV&V into your compliance protocols, you can strengthen your organization’s integrity.
If your organization uses compliance and cybersecurity tools without IV&V services, consider our Opsfolio Solutions, which inherently include IV&V services with the cybersecurity tools.
Bibliography
1. “An Assessment of Space Shuttle Flight Software Development Processes,” National Academy of Sciences, 1993
2. “ISO Standards Are Internationally Agreed by Experts,” International Organization for Standardization (ISO), 2024
3. Wolford, “What is GDPR, the EU’s new data protection law?,” GDPR.EU(Proton Technologies AG), 2024
4. “Summary of the HIPAA Security Rule,” U.S. Department of Health and Human Services (HHS), October 19, 2022
5. Swift, “Use Third-Party Validation To Build Credibility,” Forbes Media LLC., August 1, 2017
6. “Creating Value By Building Trust,” PwC India, 2024
7. “Importance of Independent Verification and Validation (IV&V) and Automated Testing for Complex IT Health Solutions,” i-Link Solutions, 2022
8. “About NASA’s IV&V Program,” National Aeronautics and Space Administration (NASA), 2024
9. “Independent Verification and Validation (IV&V) Through the Eyes of Dod,” Logapps, July 18, 2013
10. “IVV 09-1: Independent Verification and Validation Technical Framework,” National Aeronautics and Space Administration (NASA), February 26, 2016





